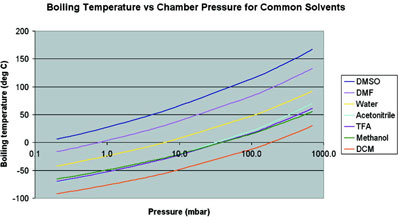
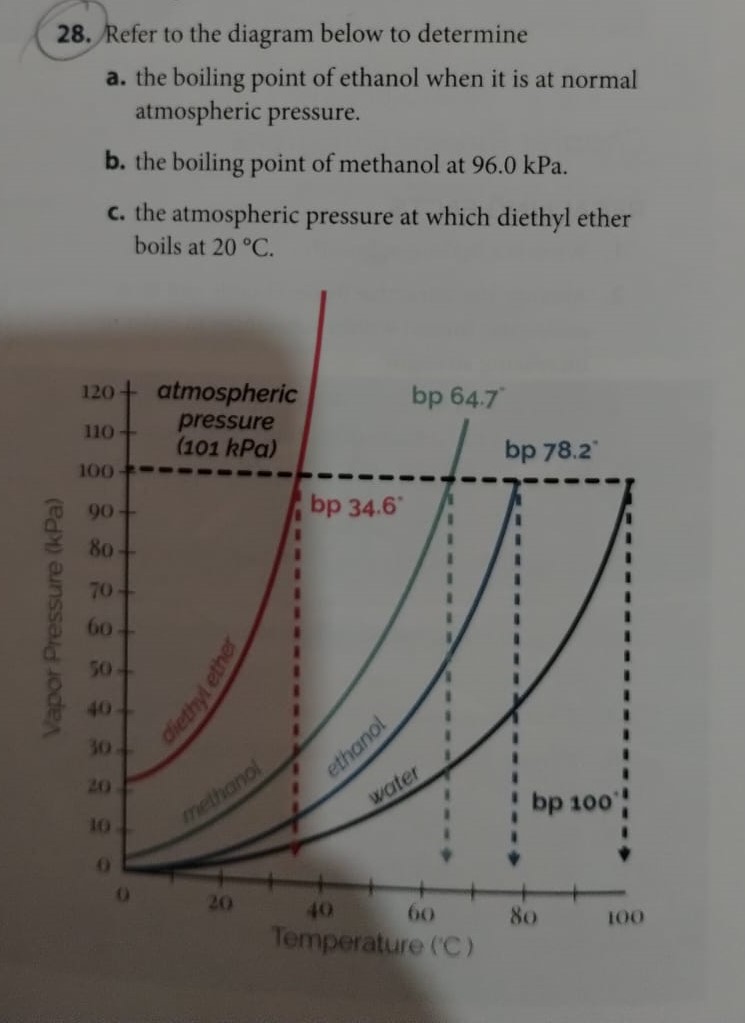
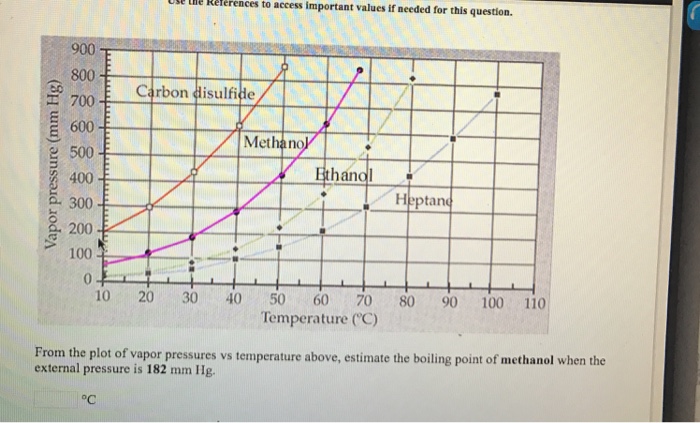
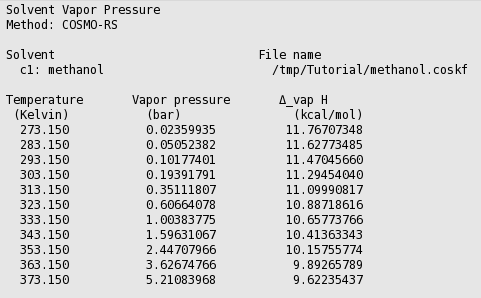
![SOLVED: 900 800 2 700 1 600 500 L 400 300 200 100 Carbon disulfide Methanol Ethanel Heptand 10 20 30 40 50 60 70 Temperature (C) 80 90 100 110 From the plot of vapor pressures vS temperature above, estimate the boiling point of ethano] when the ... SOLVED: 900 800 2 700 1 600 500 L 400 300 200 100 Carbon disulfide Methanol Ethanel Heptand 10 20 30 40 50 60 70 Temperature (C) 80 90 100 110 From the plot of vapor pressures vS temperature above, estimate the boiling point of ethano] when the ...](https://cdn.numerade.com/ask_images/541dbeec21dc4d91a0ca295324890625.jpg)
SOLVED: 900 800 2 700 1 600 500 L 400 300 200 100 Carbon disulfide Methanol Ethanel Heptand 10 20 30 40 50 60 70 Temperature (C) 80 90 100 110 From the plot of vapor pressures vS temperature above, estimate the boiling point of ethano] when the ...

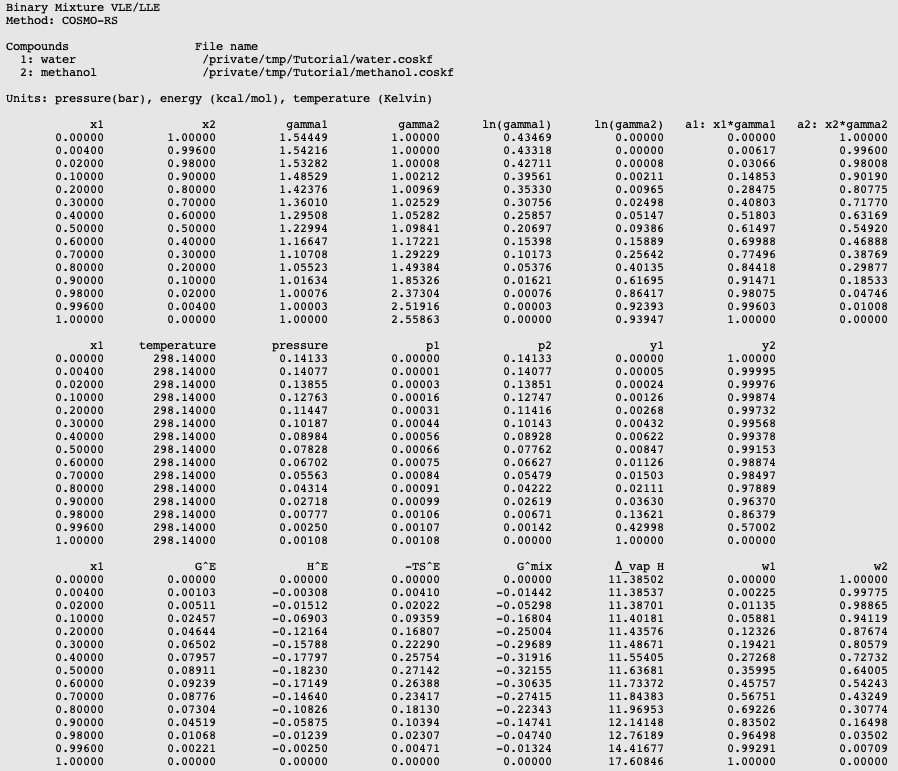
The Liquidus Temperature for Methanol‐Water Mixtures at High Pressure and Low Temperature, With Application to Titan - Dougherty - 2018 - Journal of Geophysical Research: Planets - Wiley Online Library

The vapour pressures of ethanol and methanol are `44.5` and `88.7 mm Hg`, respectively. An ideal... - YouTube
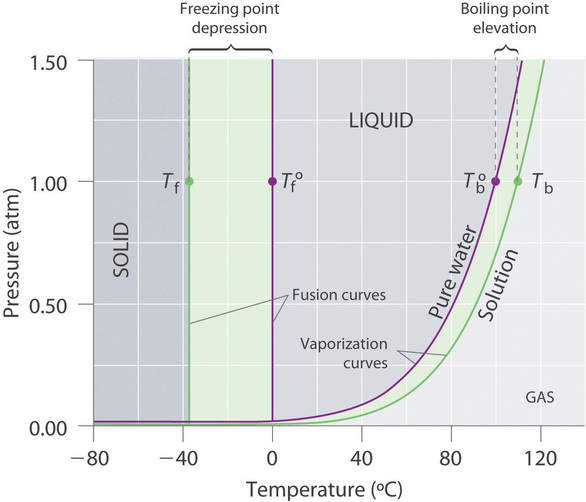
13.8: Freezing-Point Depression and Boiling-Point Elevation of Nonelectrolyte Solutions - Chemistry LibreTexts
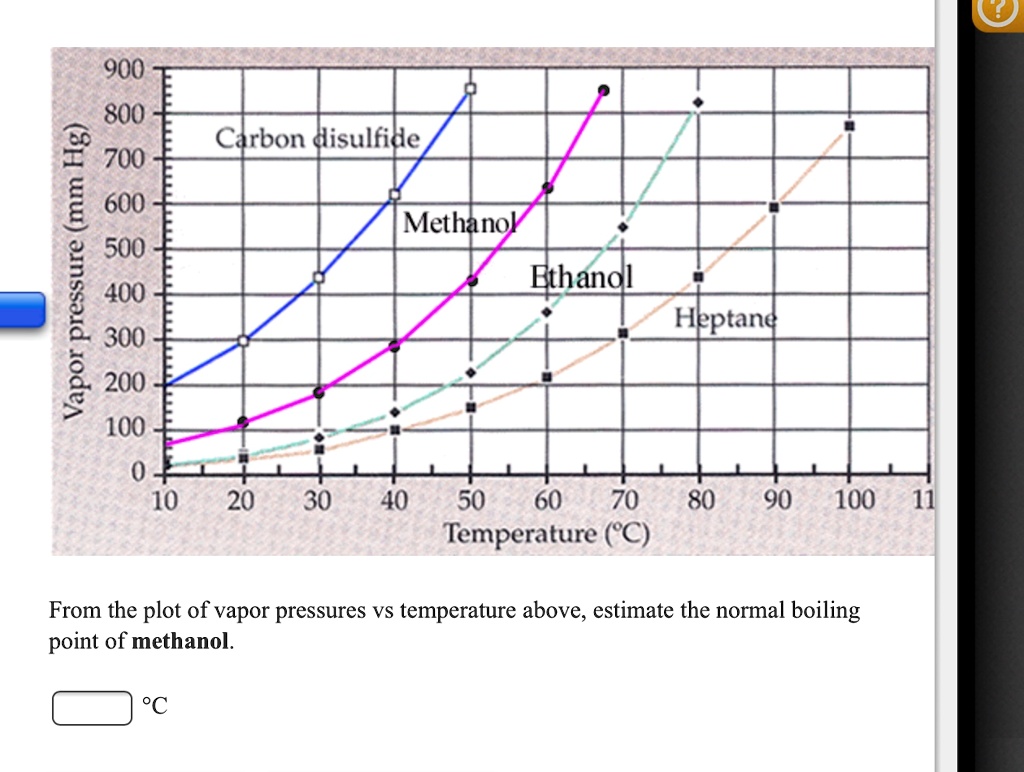
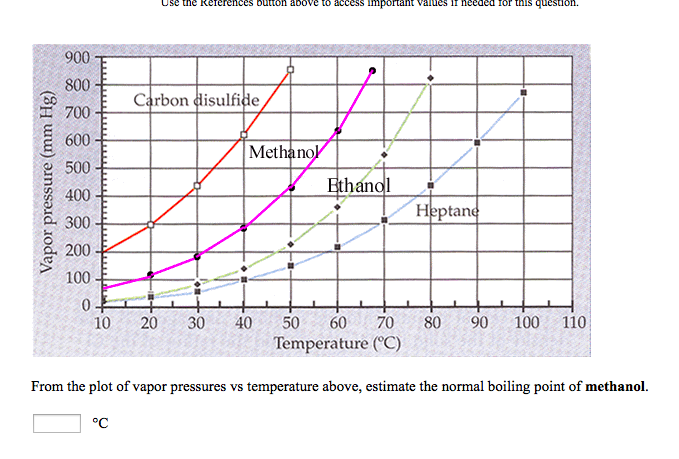
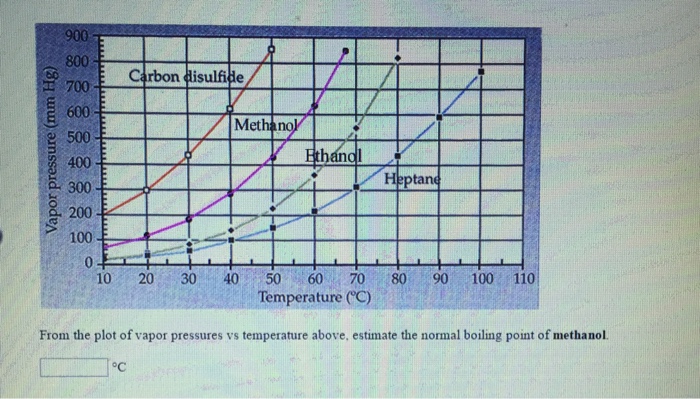
SOLVED: 900 800 2 Carbon disulfide 700 1 600 Methanok 500 L Ethanel 400 300 Heptand 1 200 100 0 10 20 30 40 50 60 80 90 100 Temperature (C) From the plot of vapor pressures vs temperature above; estimate the normal boiling point of methanol

Methanol vapor pressure curve. Markers located at atmospheric pressure... | Download Scientific Diagram

Vapor pressure of methanol and ethanol as a function of temperature... | Download Scientific Diagram

Methanol vapor pressure curve. Markers located at atmospheric pressure... | Download Scientific Diagram

A benzene-methanol system shows azeotropic behavior. After solving for the boiling points for both molecules, describe the behavior for a mixture that is initially rich in benzene (90%) and then for a
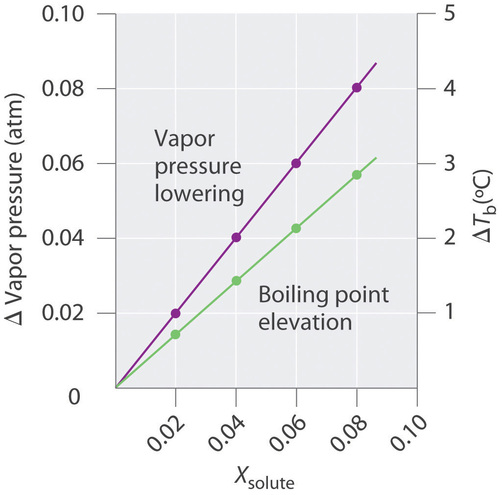
13.8: Freezing-Point Depression and Boiling-Point Elevation of Nonelectrolyte Solutions - Chemistry LibreTexts