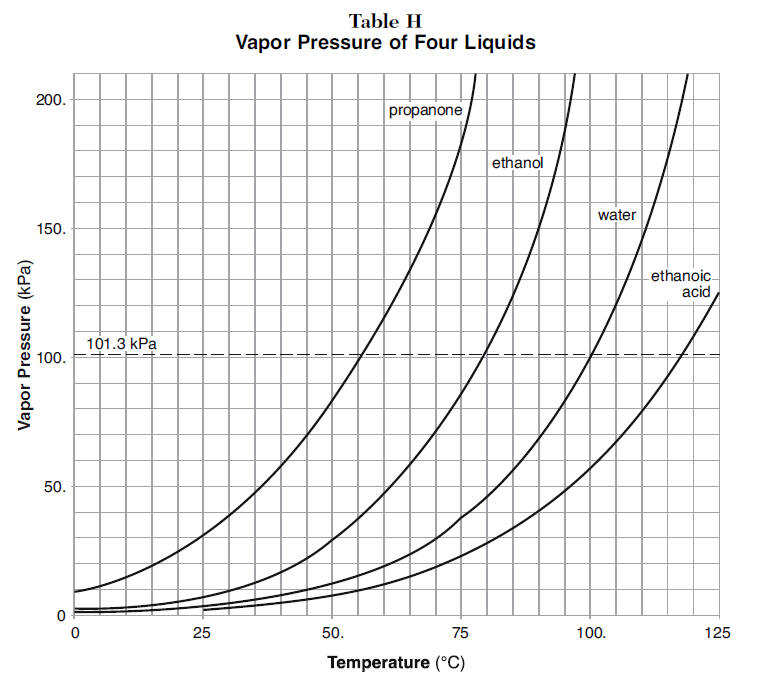
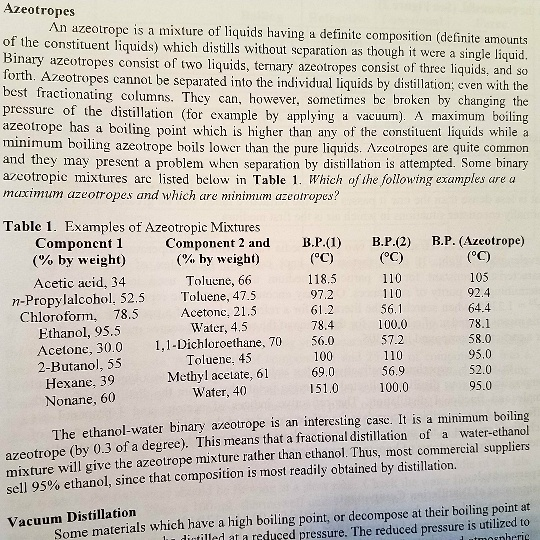
Use the figure below to determine the boiling point of -Chloroform at 80 kPa -Ethanol at 20kPa -Ethanol at - Brainly.com
![Density, Viscosity, Vapor−Liquid Equilibrium, and Excess Molar Enthalpy of [ Chloroform + Methyl tert-Butyl Ether] | Journal of Chemical & Engineering Data Density, Viscosity, Vapor−Liquid Equilibrium, and Excess Molar Enthalpy of [ Chloroform + Methyl tert-Butyl Ether] | Journal of Chemical & Engineering Data](https://pubs.acs.org/cms/10.1021/je100821g/asset/images/medium/je-2010-00821g_0003.gif)
Density, Viscosity, Vapor−Liquid Equilibrium, and Excess Molar Enthalpy of [ Chloroform + Methyl tert-Butyl Ether] | Journal of Chemical & Engineering Data

Normalized UV–vis absorption spectra in a) chloroform solution and b)... | Download Scientific Diagram

Potential Use of Limonene as an Alternative Solvent for Extraction of Gutta-Percha from Eucommia ulmoides | ACS Sustainable Chemistry & Engineering

Compact organic liquid dielectric resonator antenna for air pressure sensing using soft material | Scientific Reports

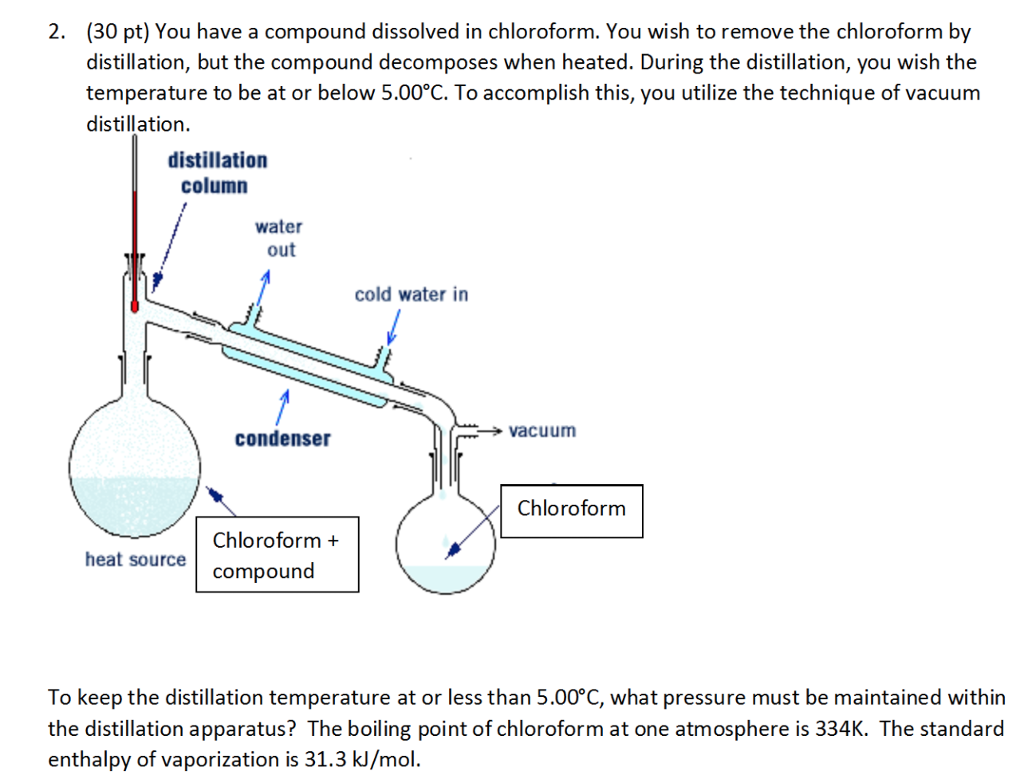
SOLVED: You have compound dissolved in chloroform and need t0 remove the solvent by distillation Because the compound is heat sensitive, you hesitate to raise the temperature above 5.00"C and decide on

Comparison of extractive distillation and pressure-swing distillation for acetone/chloroform separation - ScienceDirect

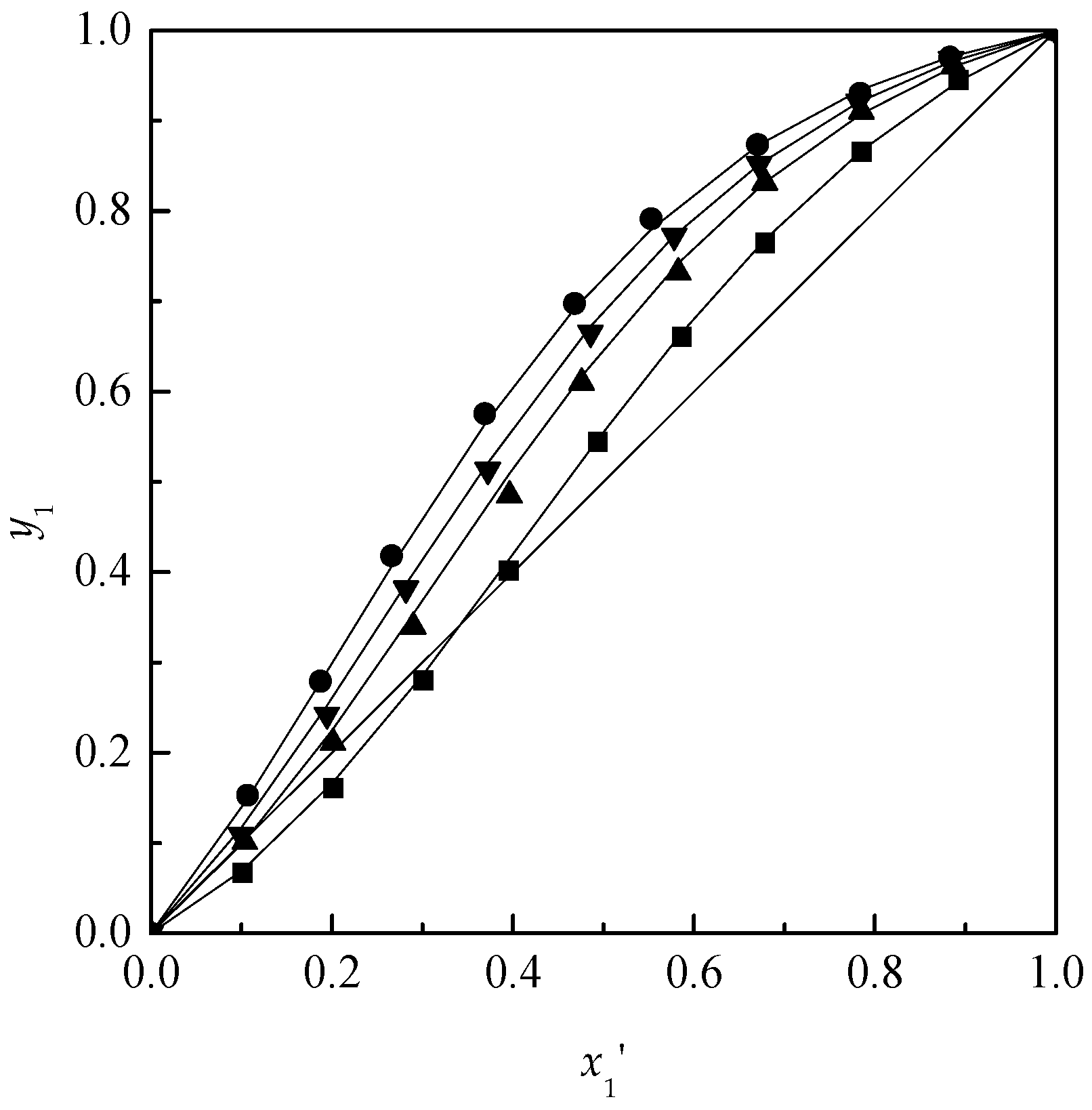
Applied Sciences | Free Full-Text | Effect of Ionic Liquids on the Isobaric Vapor-Liquid Equilibrium Behavior of Acetone-Chloroform
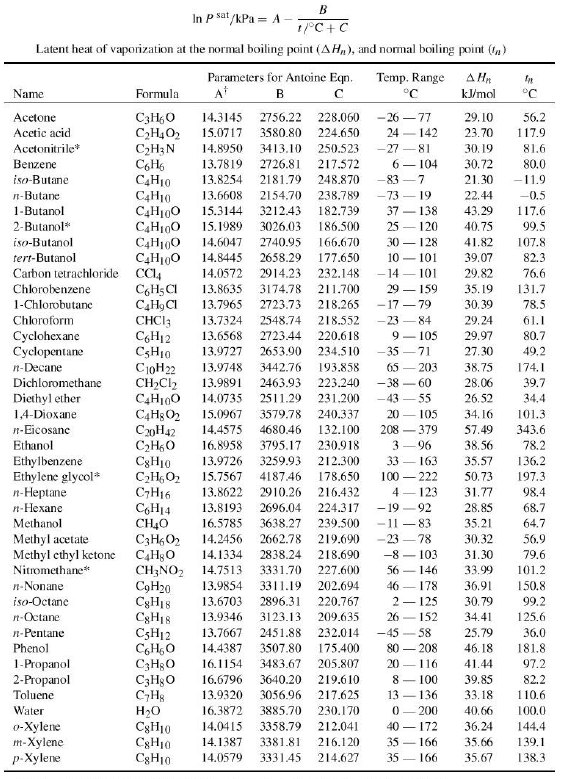
What is the normal boiling point of chloroform if its heat of vaporization is 31.4 kJ/mol and it has a vapor pressure of 190.0 mmHg at 25.0 °C? - Quora
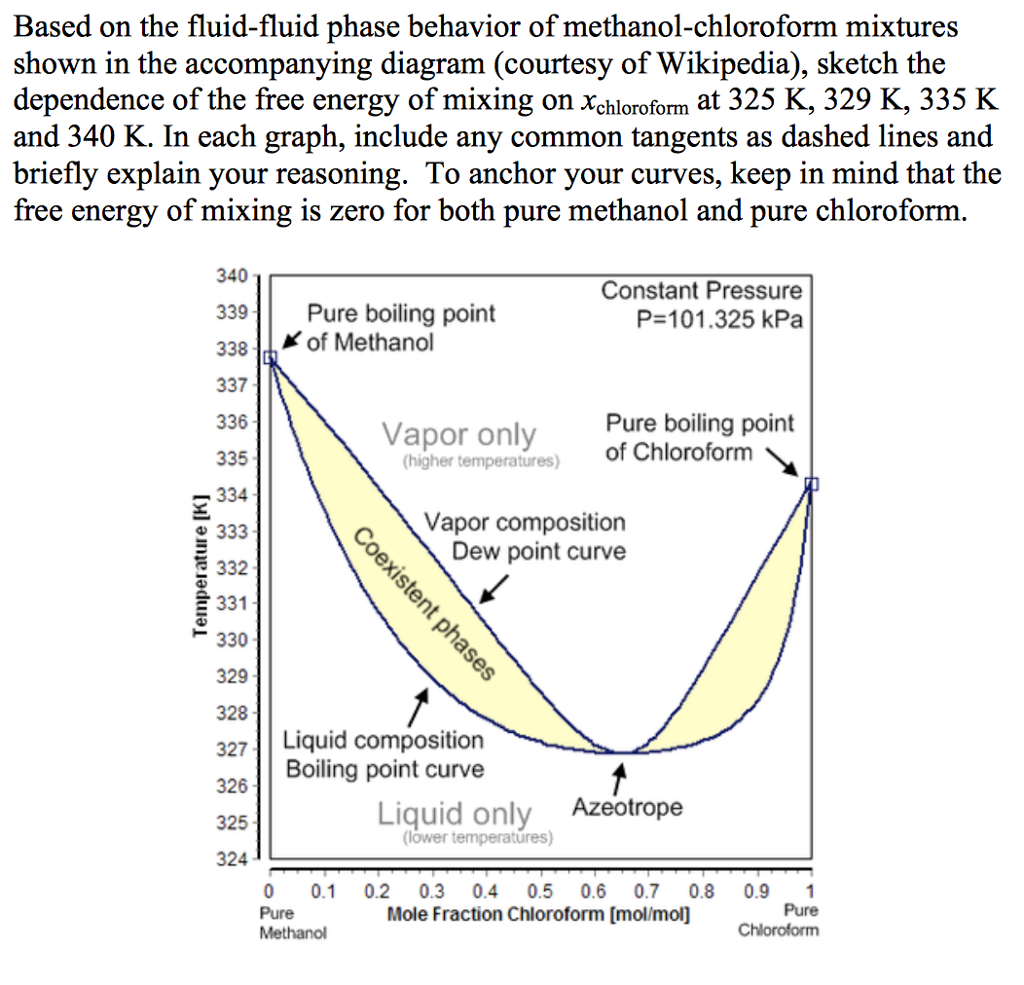
Separation of methanol-chloroform mixture using pressure-swing distillation: Modeling and optimization

Comparison of extractive distillation and pressure-swing distillation for acetone/chloroform separation - ScienceDirect