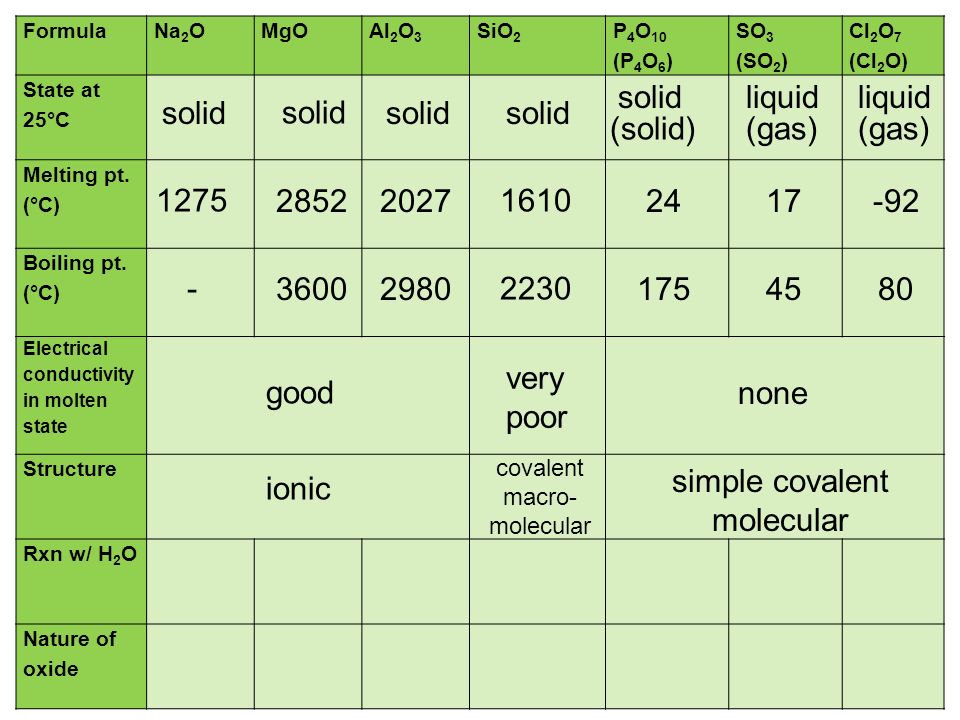
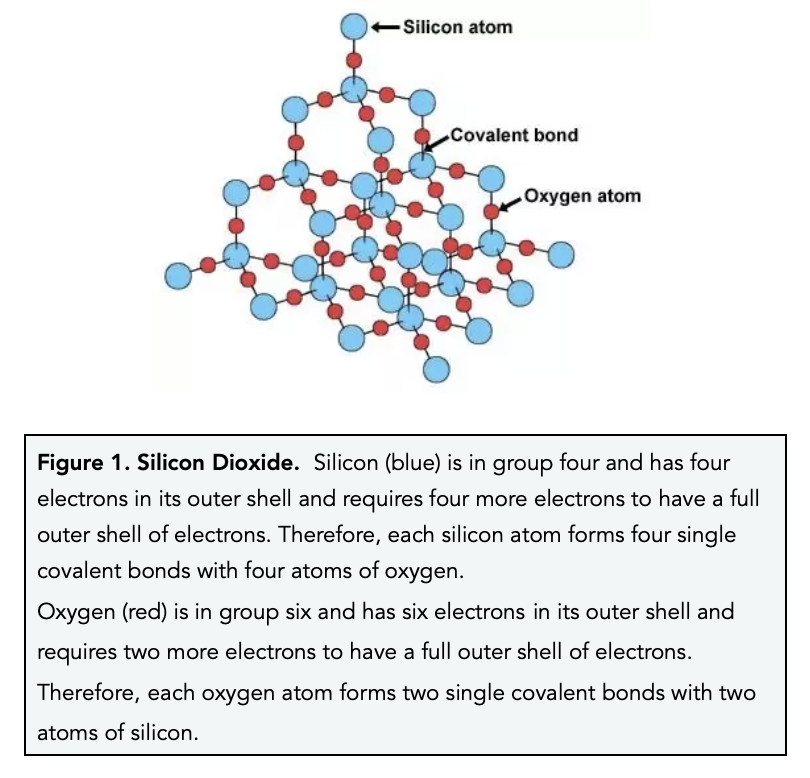
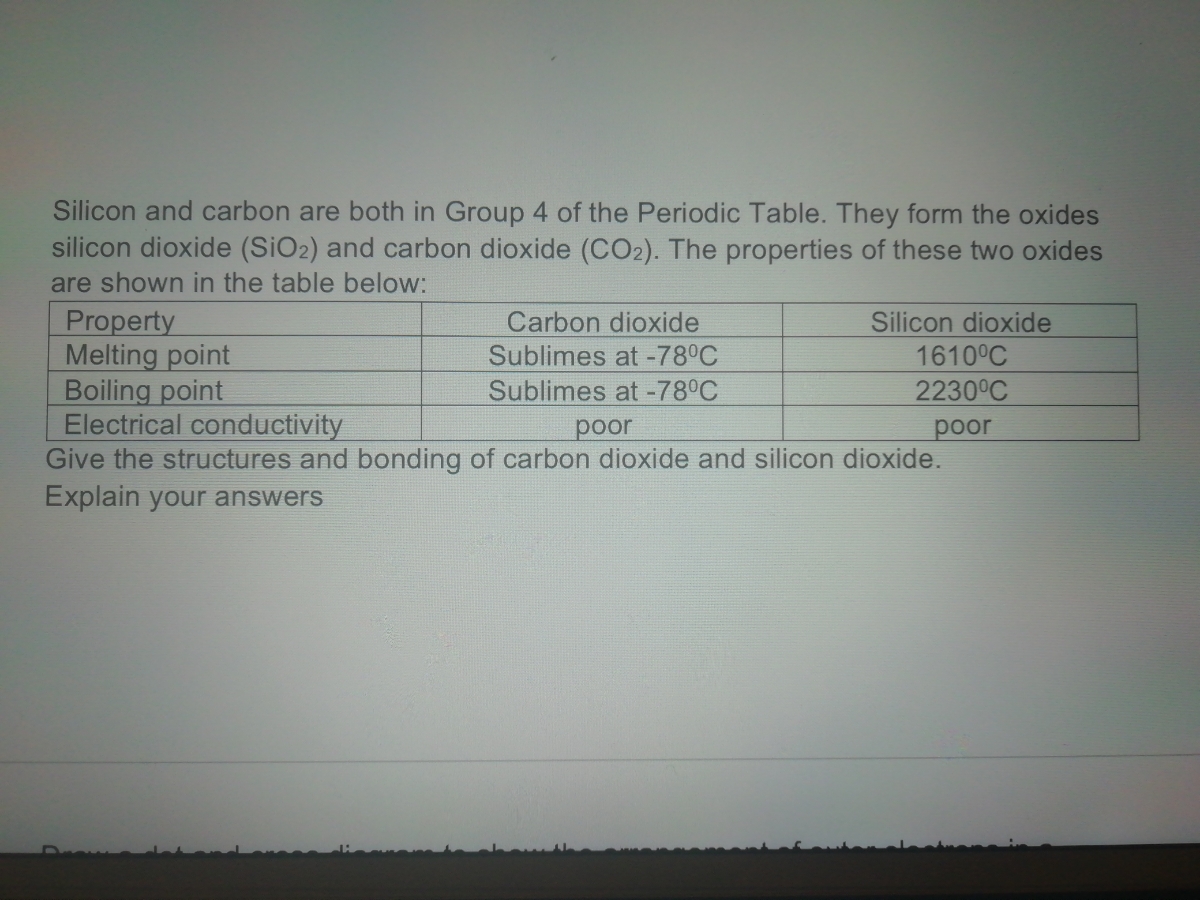
Using diagrams, explain in terms of structure and bonding why the melting point of SiO2 is much higher than that of P4O10. | Homework.Study.com
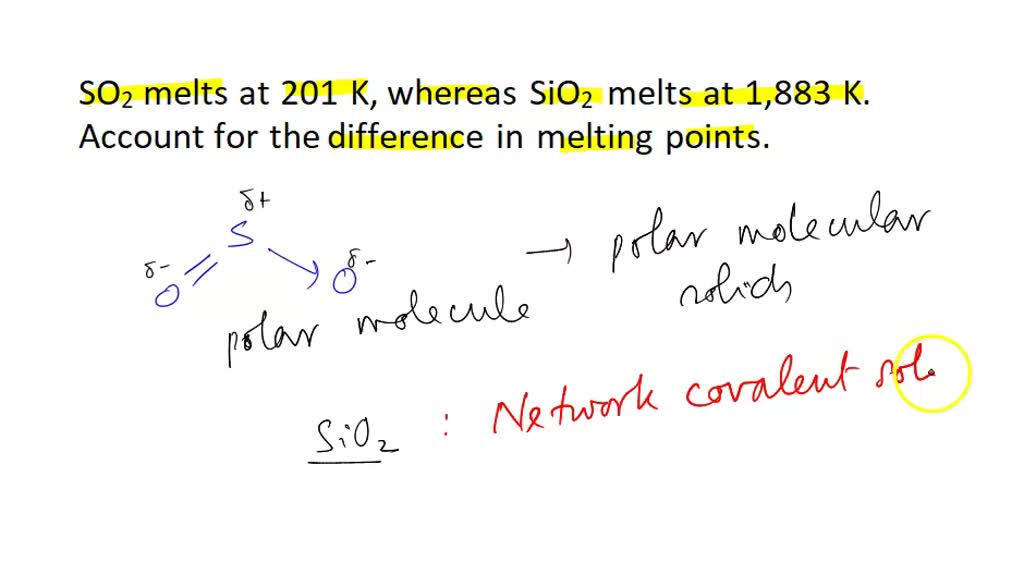
SOLVED: SO2 melts at 201 K, whereas SiO2 melts at 1,883 K. Account for the difference in melting points. You must discuss both of the substances in your answer.

Dipole Formation and Electrical Properties According to SiO2 Layer Thickness at an Al2O3/SiO2 Interface | The Journal of Physical Chemistry C