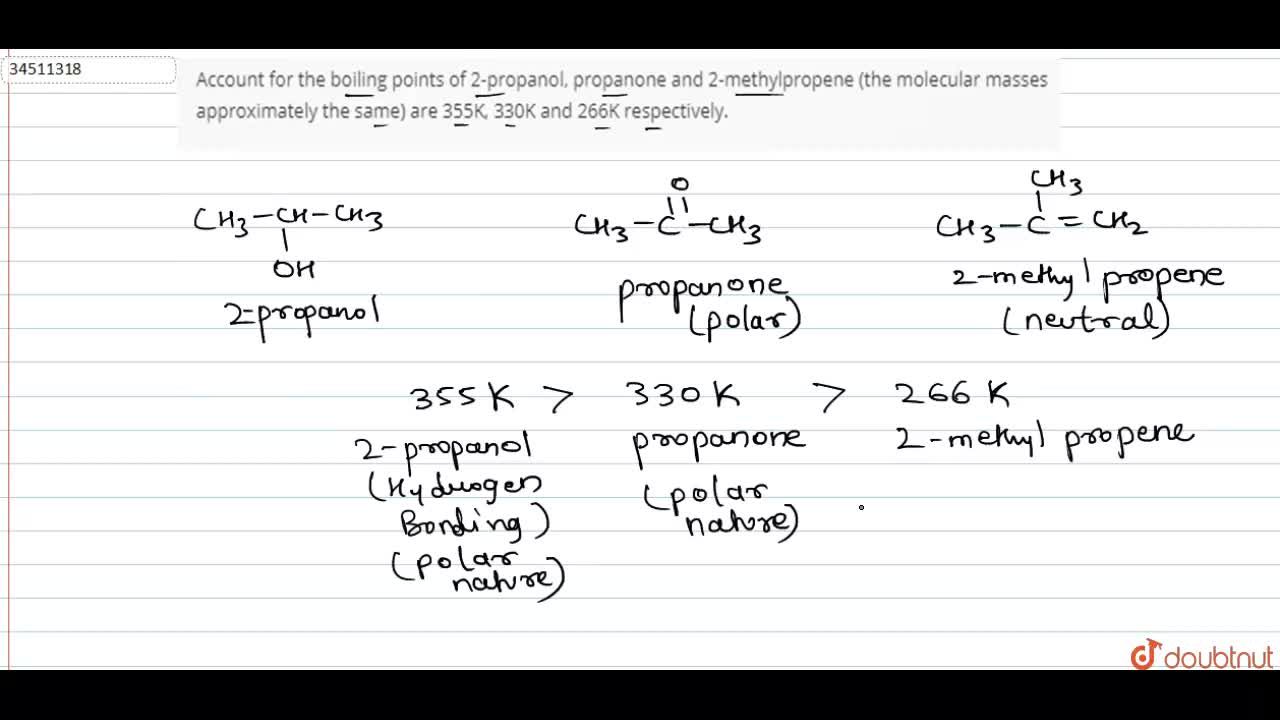
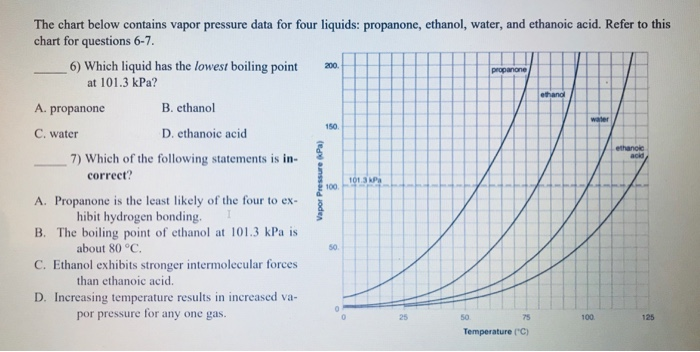
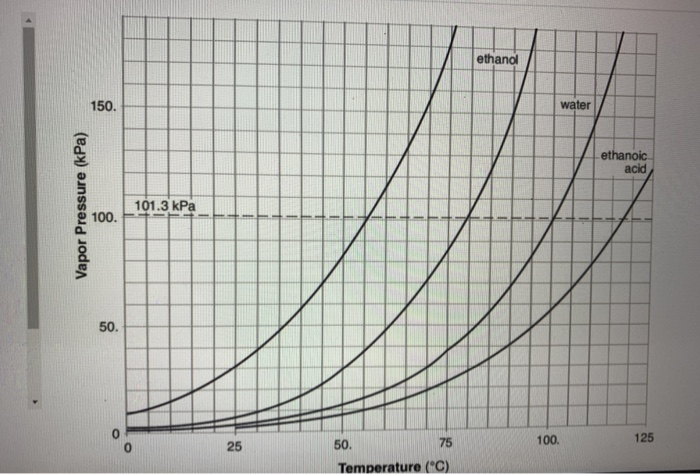
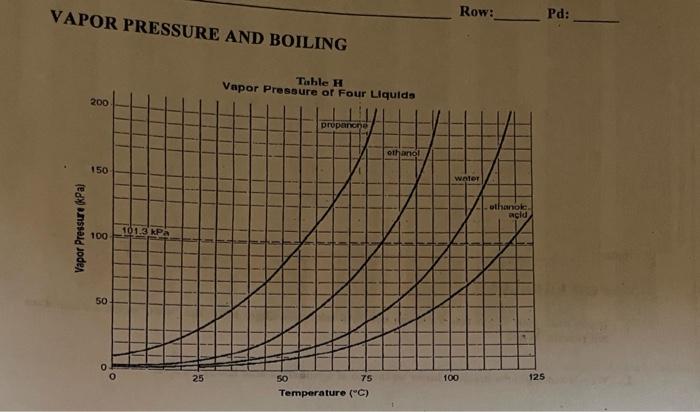
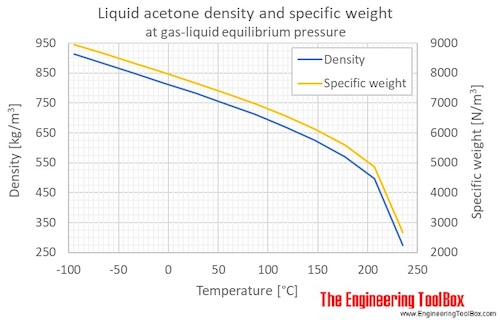
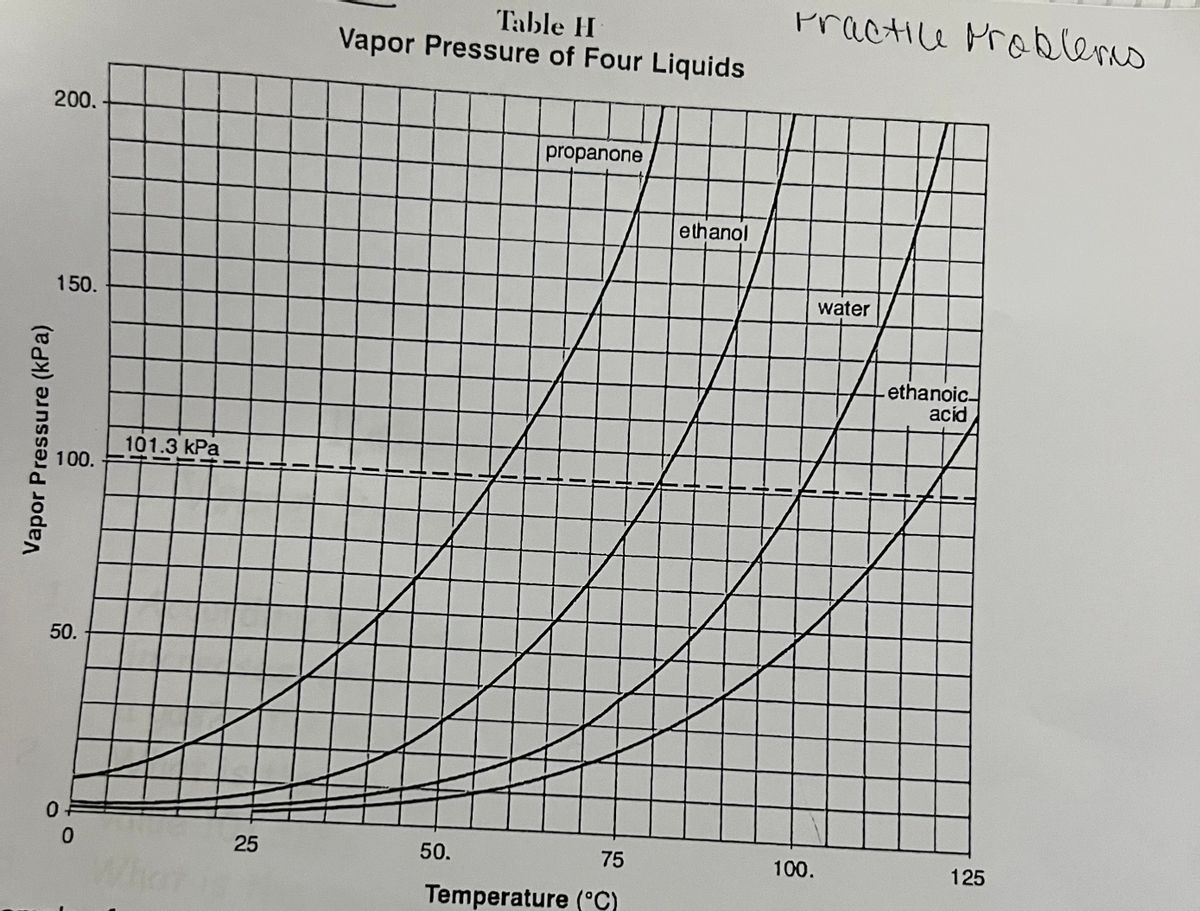
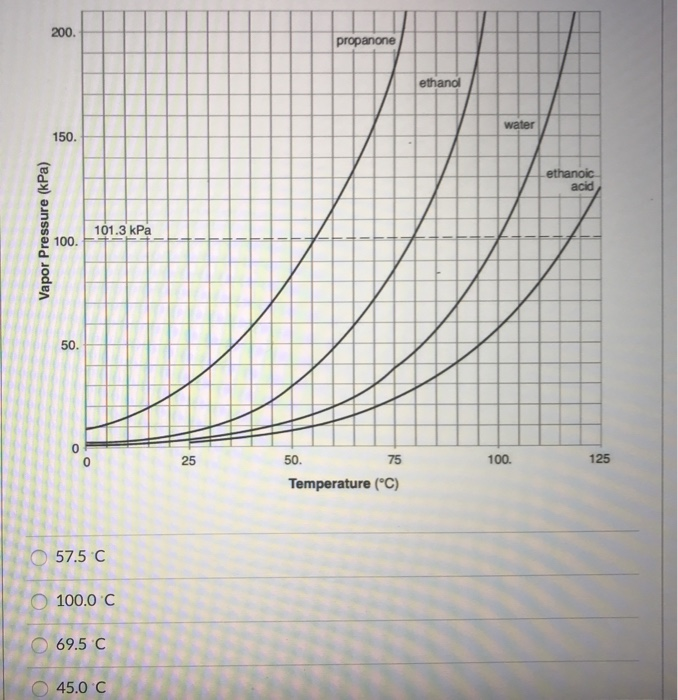
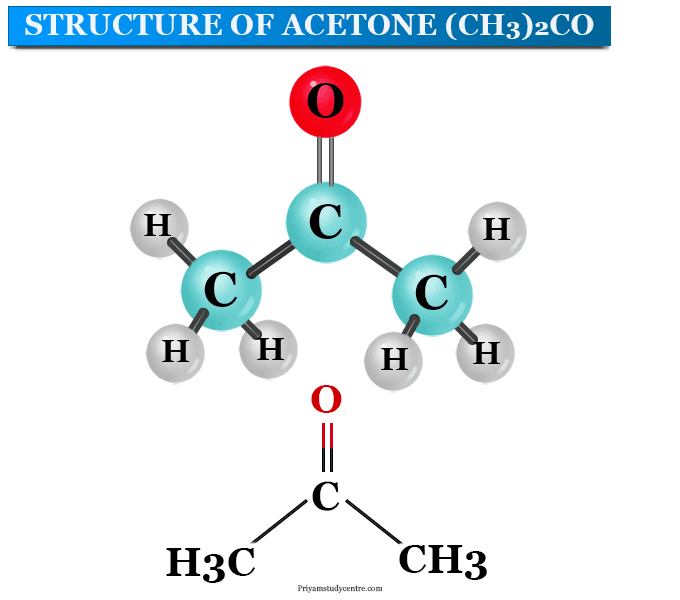
For each of the compounds below, explain why they do not have the same value for their standard heat of vaporization. 1. Propane and propanone 2. Propanone and 1-propanol | Homework.Study.com
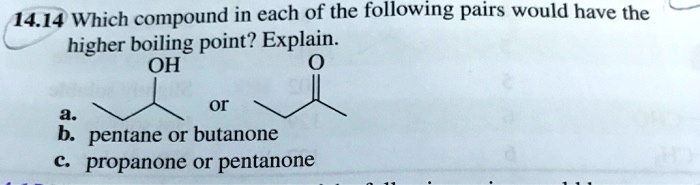
SOLVED: 14.14 Which compound in each of the following pairs would have the higher boiling point? Explain. OH or b: pentane or butanone propanone or pentanone

Cambridge International AS and A Level Chemistry Coursebook with CD-ROM by Cambridge University Press Education - Issuu

SOLVED: Which of the following has the highest boiling point? propanone 2-pentanone butanone 3-octanone

The boiling point of pure acetone is 56.38^(@)C`. When 0.707 g of a compound is dissolved in 10 g of - YouTube

SOLVED: arrange the following compounds in order of increasing boiling points: Acetone, n-butane, propanal, 1-propanol, 2-propanol. Explain the reasons for your arrangement