OneClass: rank these compounds by boiling point Rank these compounds by boiling point. Highest Boilin...

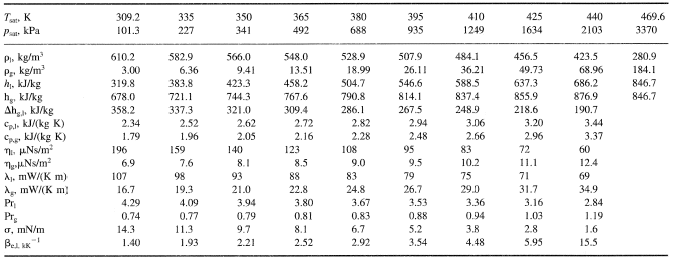
SOLVED: 1)The following information is given for n-pentane, C5H12, at 1atm: boiling point = 36.2 °C Hvap(36.2 °C) = 25.8 kJ/mol specific heat liquid = 2.28 J/g°C At a pressure of 1
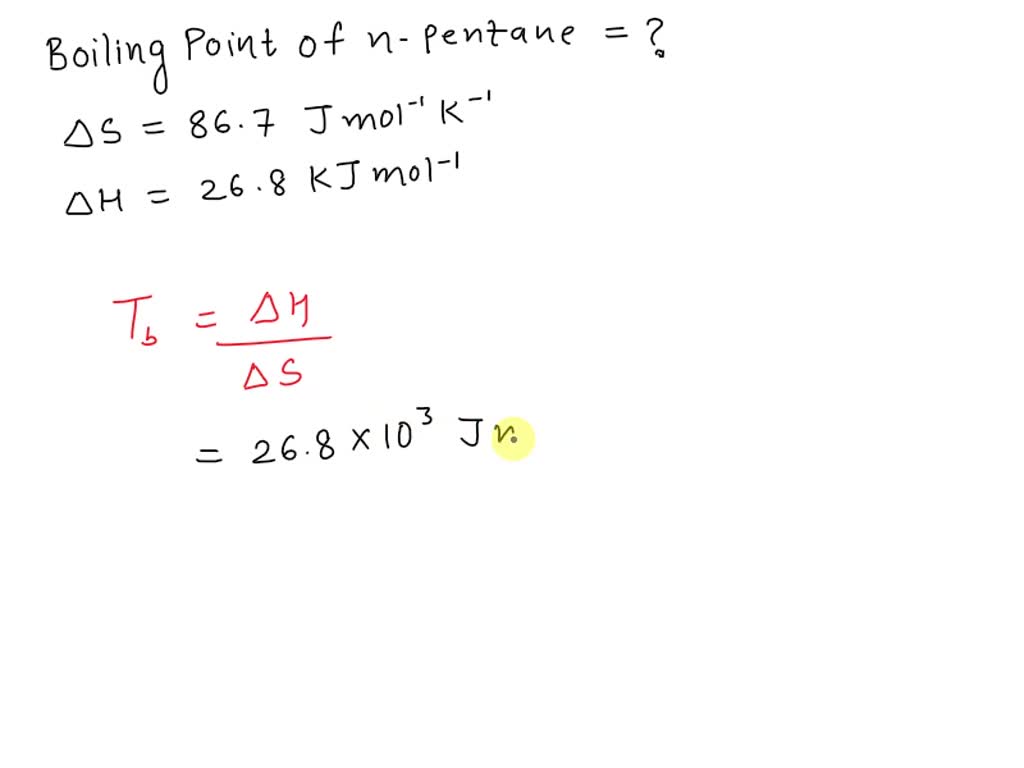
SOLVED: Calculate the boiling point (BP) of n-pentane, given that the = Points) entropy 14)(6= BPis86.7 Jlmol x *K and the change in enthalpy is 26.8 kJlmole change at the
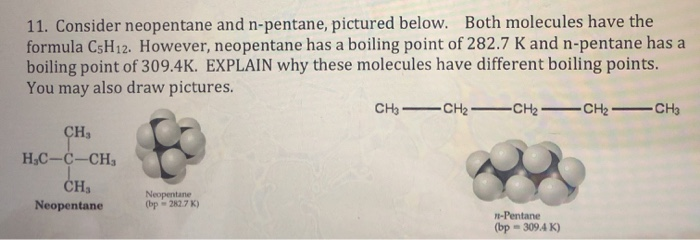
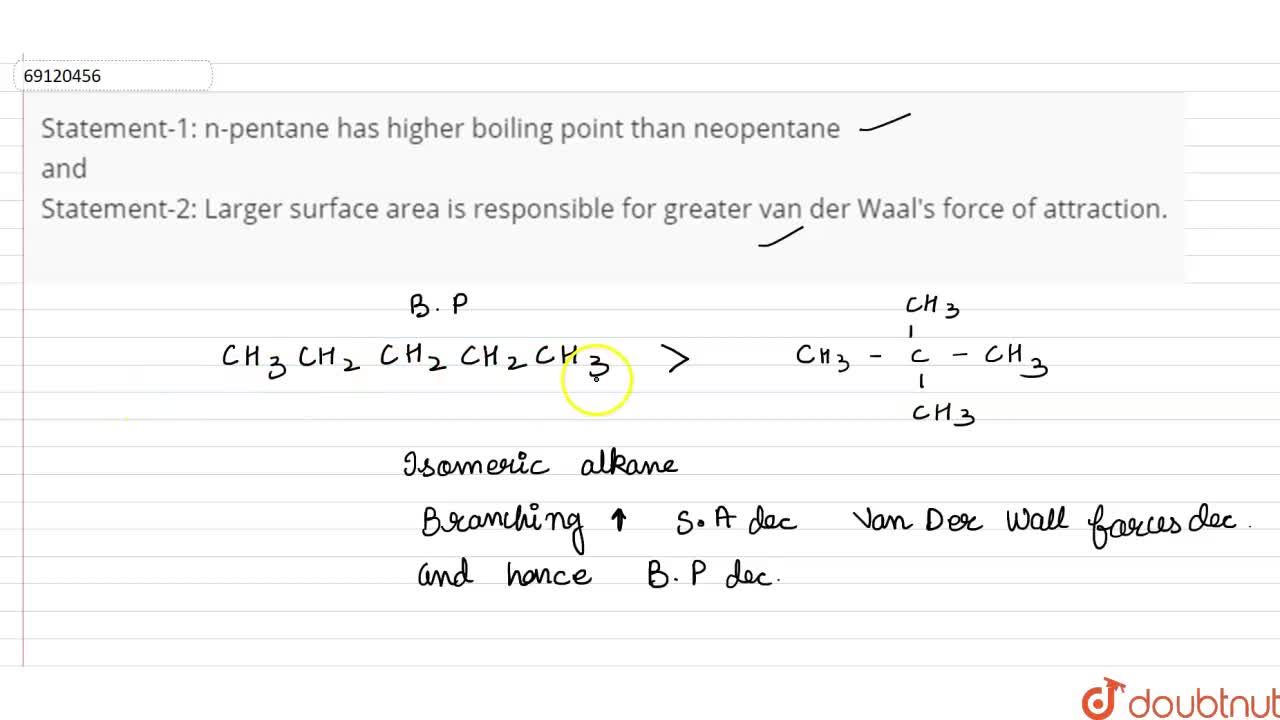
21. Why The boiling point of pentane is greater than isopentane? And why the boiling point of neopentane is less than N pentane and isopentane?
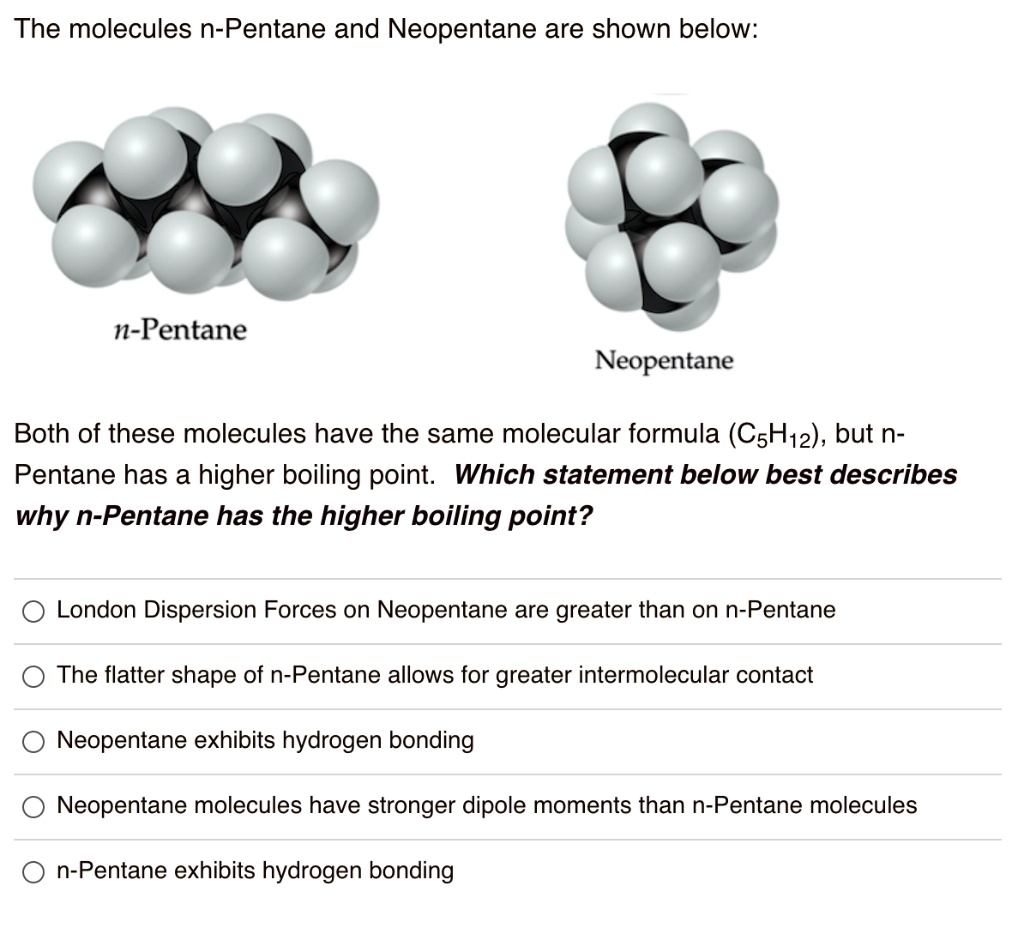
SOLVED: The molecules n-Pentane and Neopentane are shown below: n-Pentane Neopentane Both of these molecules have the same molecular formula (C5H12), but n- Pentane has a higher boiling point: Which statement below

Pentane has a boiling point of 36.1 degrees Celsius while 1-butanol, which has a similar mass, has a boiling point of 117.7 degrees Celsius. Explain this difference, including line-angle structures of each
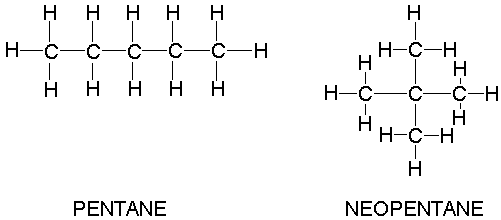
Why when the shape of molecules become more compact it's boiling point decrease while when intermolecular force become strong boiling point increase? | Socratic

Arrange the following compounds in the descending order of their boiling pointsa) n - pentaneb) isopentanec) neopentane